Nutrition Labeling
Since there is nothing added to sugar, bags and boxes of sugar are exempt from bearing a grams of added sugars declaration.
Learn More“Consumers deserve to know what is in their food so they can make informed decisions for themselves and their families,” said Courtney Gaine, PhD, RD, President and CEO of the Sugar Association. “These changes by FDA will bring the complete transparency in sweetener labeling that we know consumers want, deserve and should expect.”
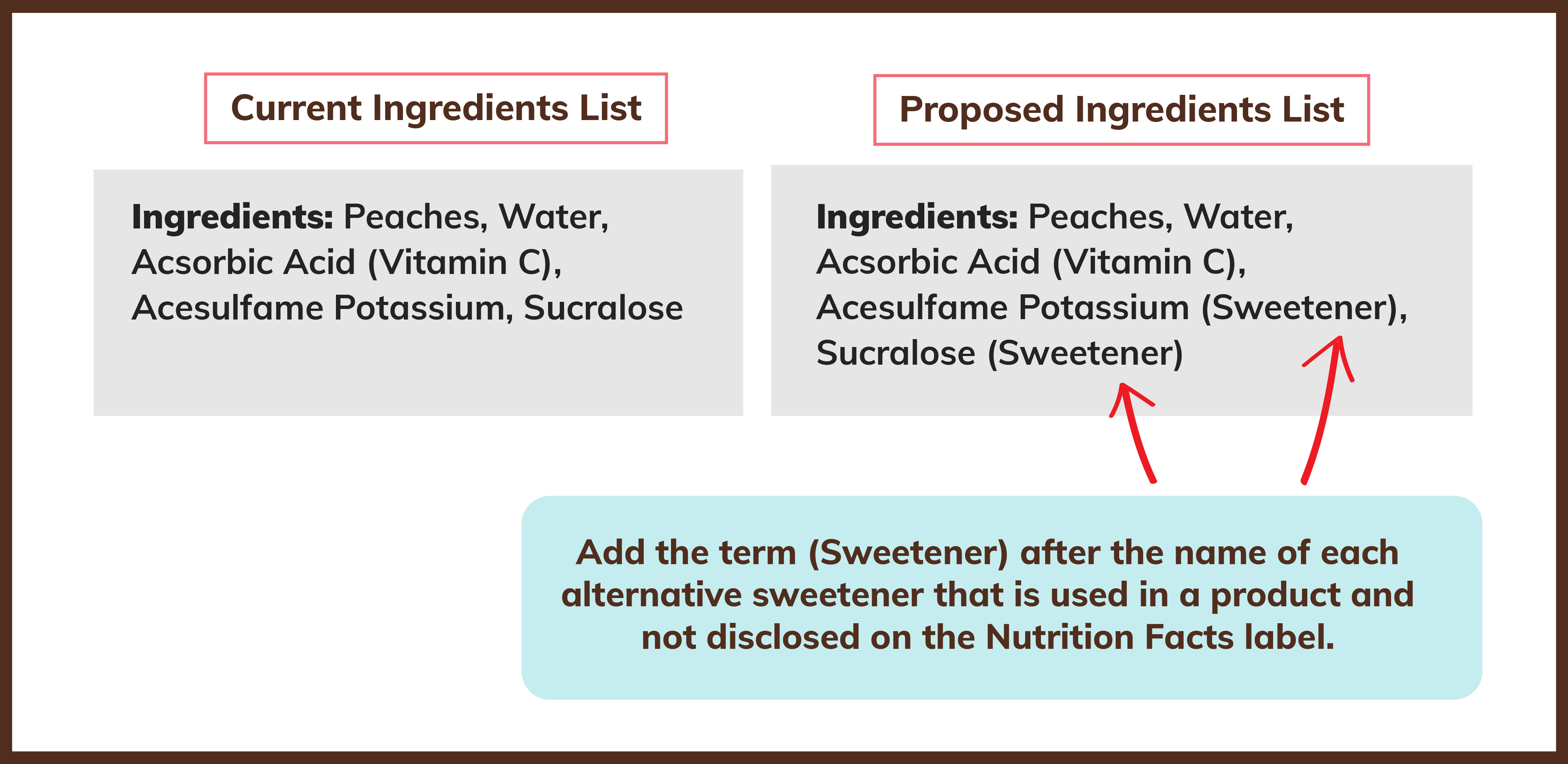
The Petition asks the FDA to require the following changes to food labeling by issuing official industry guidance supported by the Agency’s enforcement discretion:
Link to Supplement to Petition
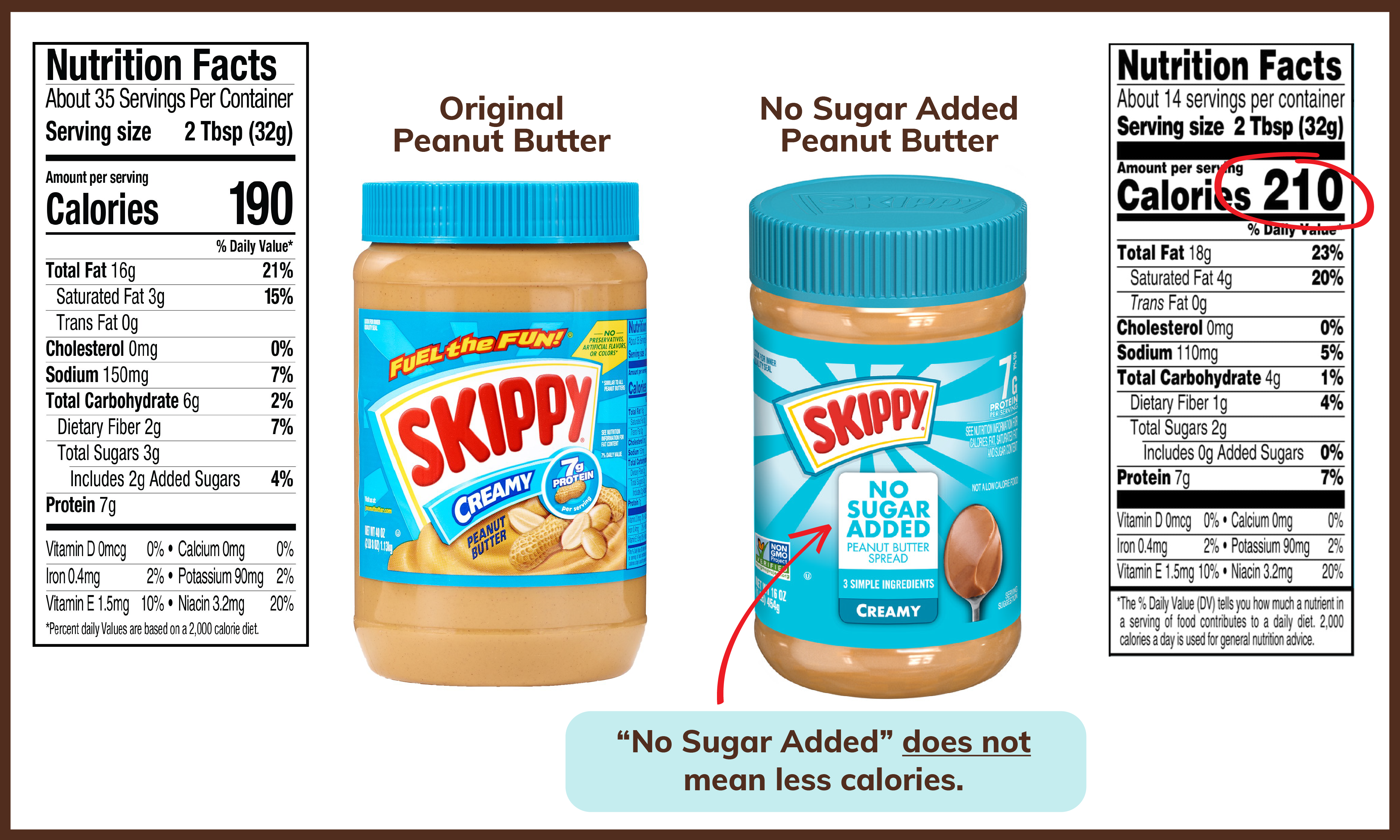
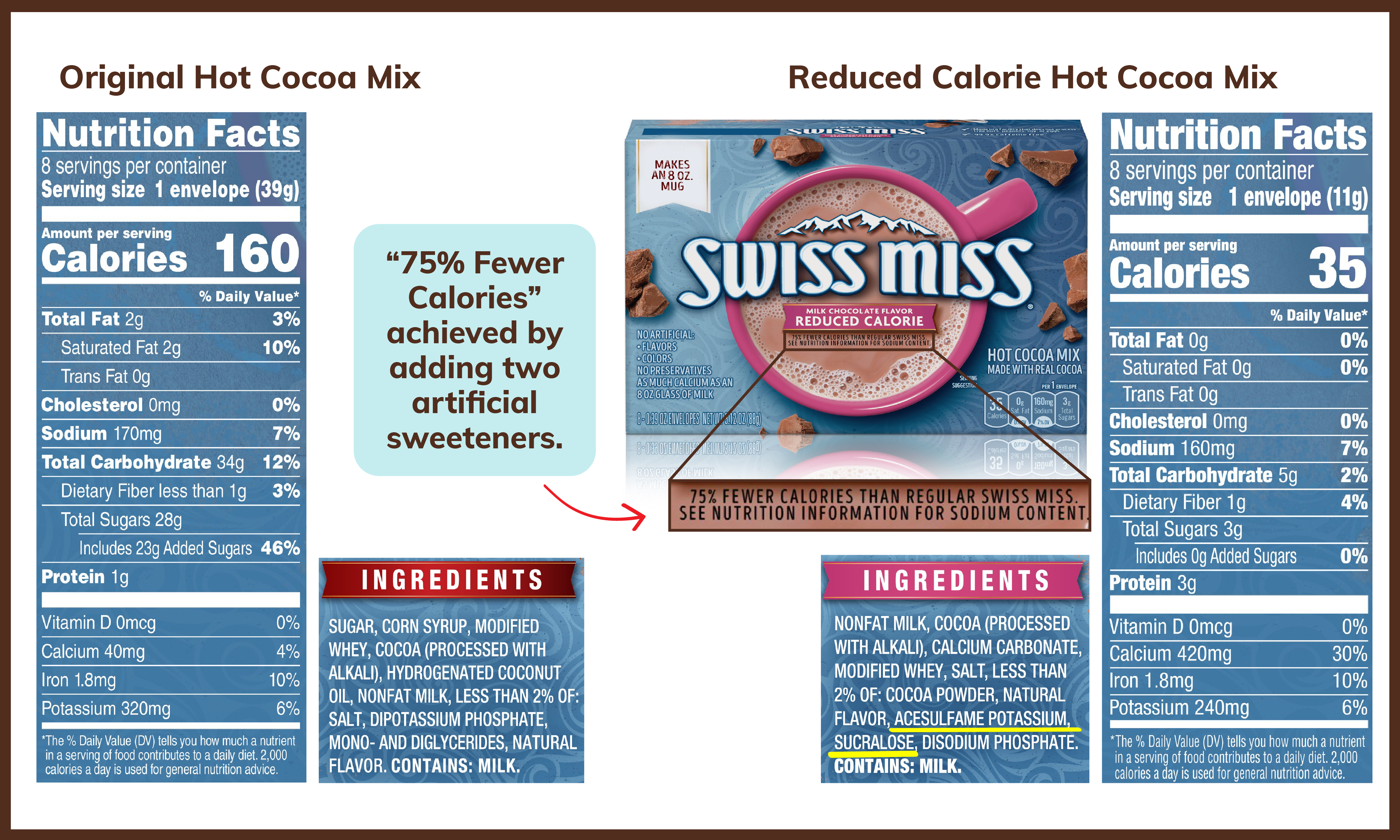
Additionally, given FDA’s new requirement to label added sugars on the Nutrition Facts label, there has been a sharp increase in the use of alternative sweeteners in packaged food, unbeknownst to many consumers. There has also been an increase in the marketing and use of sugar content claims with foods containing alternative sweeteners.
As it stands, we have an alternative sweetener labeling scheme that is incomplete, lacks transparency and is misleading and confusing to consumers. Our proposed changes will provide consumers with clear and accurate information about the use of low- and no- calorie sweeteners in consumer products and help them make more informed decisions for themselves and their families.
In March 2022, we submitted a Supplement to the original Citizen Petition presenting new supporting information and data.
Consumers, food companies and other interested parties can continue to provide comments to FDA in support of our request. Comments can be submitted to the docket, FDA-2020-P-1478. Once FDA has the opportunity to complete their review of the Petition and if changes are determined necessary, the agency will set a date when food and beverage manufacturers must comply with new labeling changes.
1. Baker-Smith CM, et al. American Academy of Pediatrics, Committee on Nutrition. Pediatrics. 2019;144(5):e20192765.










Since there is nothing added to sugar, bags and boxes of sugar are exempt from bearing a grams of added sugars declaration.
Learn More© 2024 The Sugar Association, Inc. All rights reserved.
Get Social with #MoreToSugar